OrciVita provides end-to-end Ethics Committee (ECMS) registration, re-registration, and management services to hospitals, institutions, and research centers across India. Our services are designed to ensure full compliance with NDCTR 2019, ICMR Ethical Guidelines, and ICH-GCP requirements, while reducing administrative and operational burden for institutions.
OrciVita supports hospitals and institutions seeking to establish their Institutional Ethics Committee (IEC) from inception. Our services include:
For institutions with already registered ECs, OrciVita offers comprehensive EC re-registration and ongoing management support, including:
OrciVita has successfully supported multiple EC registrations and re-registrations, including:
Currently, multiple EC registration and re-registration projects are ongoing, with OrciVita providing comprehensive regulatory and documentation support.
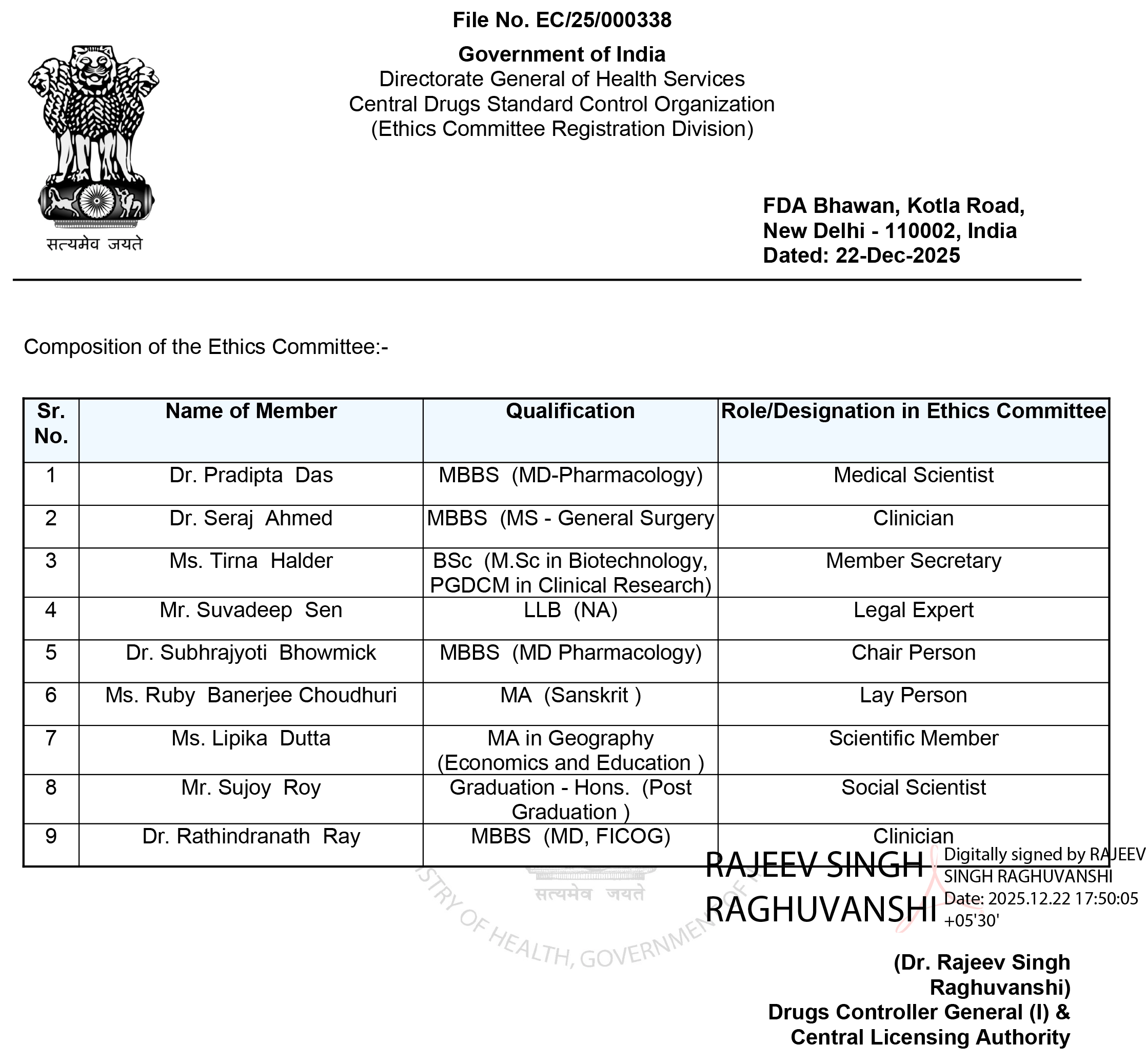
In addition to providing EC services, OrciVita has its own Independent Ethics Committee (IEC), which functions as an autonomous and unbiased ethical review body. Importantly, this IEC reviews only those clinical trials in which OrciVita is not involved, ensuring independence and avoidance of conflict of interest.
The OrciVita Independent EC operates strictly in accordance with NDCTR 2019, ICMR Ethical Guidelines, and ICH-GCP, with clearly defined SOPs, quorum requirements, and conflict-of-interest management processes.
Get in touch with us: iec@orcivita.com
The OrciVita Independent IEC is constituted as per regulatory requirements, comprising medical, non-medical, scientific, non-scientific, legal, and social representatives to ensure balanced and ethical decision-making.